Cuenta
0
0
Ningún producto
A determinar
Transporte
0,00 €
Total
Los precios se muestran con impuestos excluidos
Producto añadido correctamente a su carrito de compra
Cantidad:
Total:
Hay 0 artículos en su carrito.
Hay 1 artículo en su carrito.
Total productos:
(impuestos excl.)
Total envío (impuestos excl.):
A determinar
Total
(impuestos excl.)
:
Productos más vistos
 Ver más grande
Ver más grande
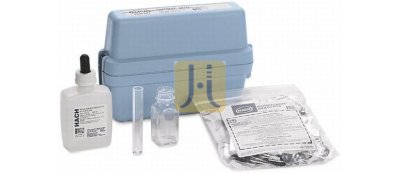
KIT DUREZA TOTAL rango 1-30 gpgp Model E-EP. HACH
RES FHS 1/8W 1. %536. KOHM
01145400
Nuevo producto
- Compartir :
Hardness:
- Water hardness is caused almost entirely by calcium and magnesium ion. Other di- and trivalent metals have a similar effect, but usually are not present in high enough concentration in potable waters to cause problems. Hardness increases soap consumption in laundries and causes scale in boilers.
- Simply count the number of drops required to change the solution color to determine hardness concentration.









